Share and Follow

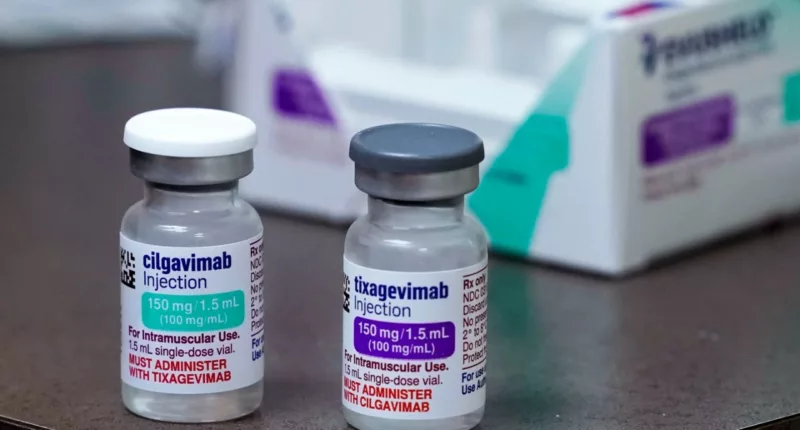
Evusheld, the preventative monoclonal antibody treatment for COVID-19, has lost its emergency use authorization in the U.S. as it is most likely not effective against the strains of the coronavirus currently circulating.
The announcement from the Food and Drug Administration (FDA) comes weeks after the agency issued a notice saying it did not expect Evusheld to be effective against the XBB.1.5 omicron subvariant, responsible for 61 percent of cases in the country, according to the most recent federal data.
“Today’s action to limit the use of Evusheld prevents exposing patients to possible side effects of Evusheld such as allergic reactions, which can be potentially serious, at a time when fewer than 10% of circulating variants in the U.S. causing infection are susceptible to the product,” the FDA said in a statement.
Evusheld is a combination of two monoclonal antibodies. It was authorized for use in people who are moderately or severely immunocompromised and likely would not have developed a strong immune response from COVID-19 vaccines.
According to a press release from Evusheld producer AstraZeneca, laboratory data has shown the treatment to be ineffective against many omicron subvariants, including the top three circulating in the U.S.: XBB.1.5, BQ.1.1 and BQ.1.
Read Related Also: Haiti receives its first batch of cholera vaccines to tackle deadly outbreak | Global health
The constant mutation of the coronavirus has rendered other similar treatments effectively obsolete. The FDA in November paused authorization on the monoclonal antibody treatment bebtelovimab, the last such treatment meant to treat coronavirus infections.
In light of these treatment becoming unavailable, the FDA advised that patients who develop symptomatic cases of COVID-19 seek out treatments such as Paxlovid, remdesivir or molnupiravir, antivirals that are still expected to be effective in treating infections.
Facilities that have Evusheld in stock, however, should retain what they have instead of disposing of the drugs, the FDA advised, as other COVID-19 strains susceptible to the treatment may become prevalent in the future.
AstraZeneca said trials are already underway on the “next-generation long-acting antibody.” According to the company, lab studies have shown its current drug candidate to be effective against all COVID-19 strains tested to date. The drugmaker is aiming to have this medication available in the later half of 2023.