Share and Follow
TAMPA, Fla. (WFLA) — For the first time, scientists have successfully treated someone suffering from Huntington’s disease, a fatal genetic illness with no cure.
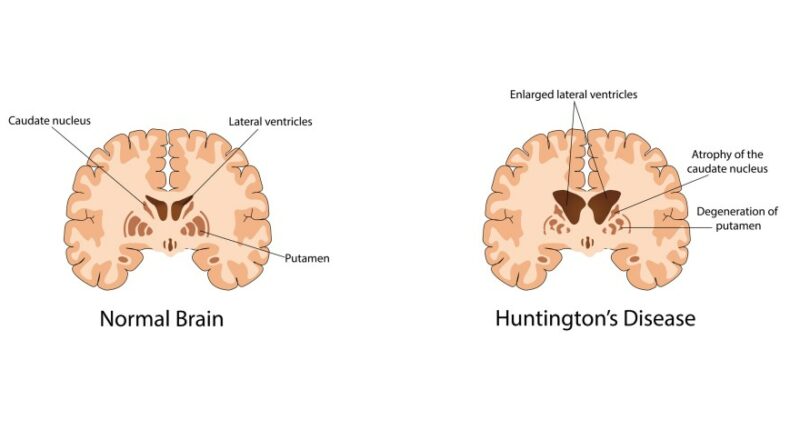
The Mayo Clinic reports that the condition causes nerve cells in the brain to decay over a period of time, causing a decline in motion, cognitive abilities and mental health. It typically manifests when someone enters their 30s or 40s, but it can also begin in patients under 20 years of age, who are diagnosed with juvenile Huntington’s disease.
According to a press release, gene therapy company uniQure announced positive results from a study of a gene therapy known as AMT-130.
The therapy works through a neurosurgical procedure that infuses AMT-130 three times on each side of the brain.

The gene therapy then uses a non-disease causing viral vector that delivers “gene encoding a microRNA” that will lower the levels of the huntingtin protein in the brain. By doing so, it reduces the level of normal huntingtin protein and the mutagenic huntingtin proteins that cause Huntington’s disease.
On Sept 24, uniQure found that patients who had high-dose AMT-130 had the progression of their Huntington’s significantly slowed by 75% at 36 months on the composite Unified Huntington’s Disease Rating Scale and 60% on Total Functional Capacity.
“I am thrilled that this pivotal study of AMT-130 showed statistically significant effects on both cUHDRS and TFC at 36 months, supported by mean CSF NfL remaining below baseline,” stated Sarah Tabrizi, M.D., FRCP, FRS, FMedSci, Ph.D., professor of clinical neurology, director of the University College London Huntington’s Disease Center and joint head of the department of neurodegenerative disease. “I believe these groundbreaking data are the most convincing in the field to date and underscore potential disease-modifying effects in Huntington’s disease, where an urgent need persists. These data indicate that AMT-130 has the potential to meaningfully slow disease progression – offering long-awaited hope to individuals and families impacted by this devastating disease.”
Those with high-dose AMT-130 appeared to have better outcomes compared to those with low-dose AMT. So far, the gene therapy drug has been found to be “generally well-tolerated” with no new serious side effects seen since December 2022, according to data as recent as June 30, 2025.
“We are incredibly excited about these topline results and what they may represent for individuals and families affected by Huntington’s disease,” stated Walid Abi-Saab, M.D., chief medical officer of uniQure. “These findings reinforce our conviction that AMT-130 has the potential to fundamentally transform the treatment landscape for Huntington’s disease, while also providing important evidence supporting one-time, precision-delivered gene therapies for the treatment of neurological disorders.”
Prior to this finding, treatments for Huntington’s disease only helped with the symptoms of movement and mental health problems. None actually combatted the condition itself before AMT-130.

Huntington’s disease is caused by a dominant gene that causes the disorder and is passed from parent to child. Every child has a 50% chance of inheriting the condition from a parent with Huntington’s.
Preventing the disease from being passed on can be done with family planning options, genetic testing, and in vitro fertilization to select specific embryos without the Huntington’s gene.