Two scientists with limited funds, extraordinary dedication and impressive scientific skills are on the brink of making a life-enhancing change to the treatment of cancer. Watch The Cancer Killers on SBS On Demand.
Lois Harris shared her battle with cancer, saying, “It has spread everywhere — my neck, ribs, liver, and small intestine. I’ve never been led to believe they could cure it,” during an interview with Insight.
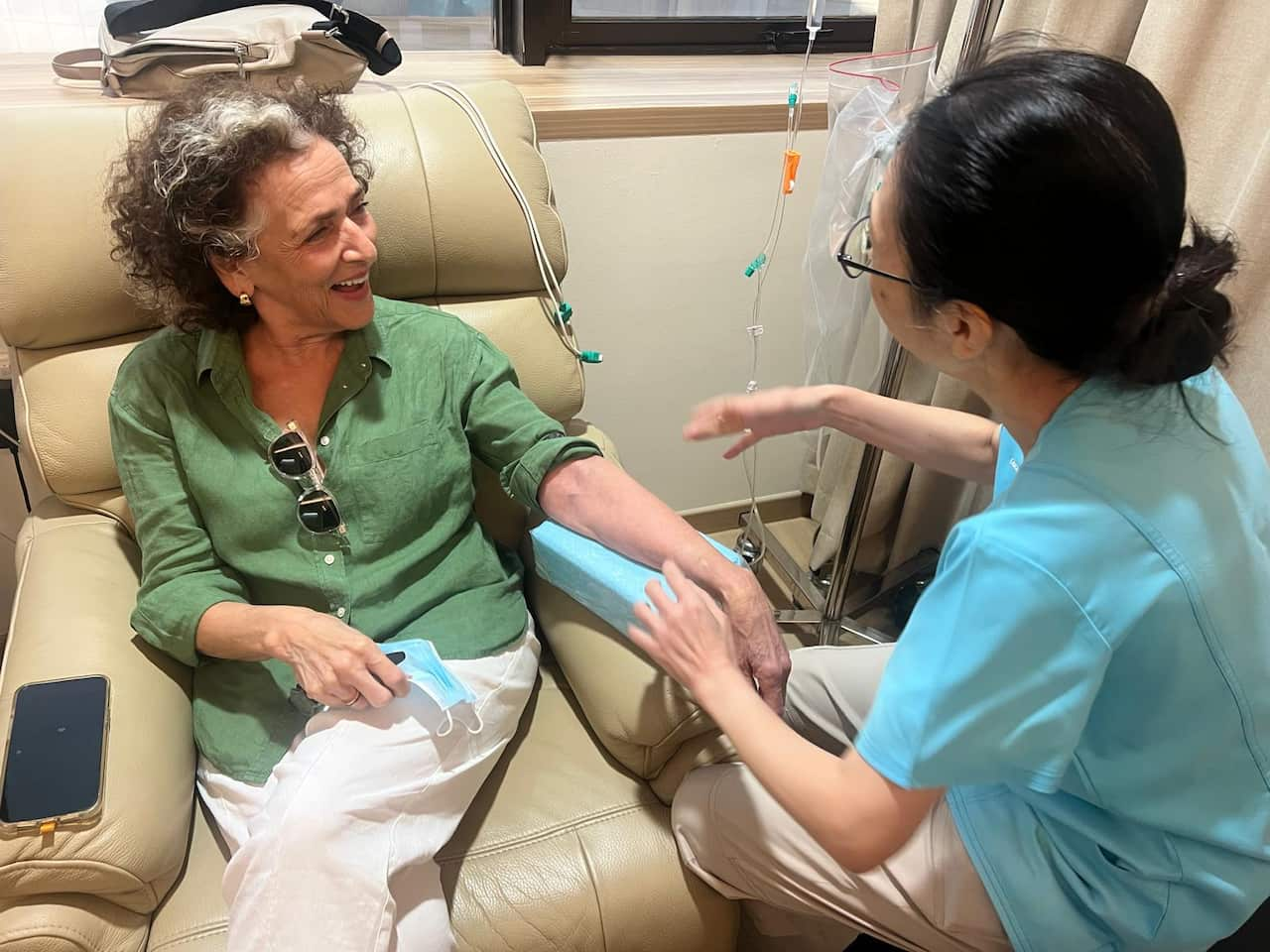
Despite the grim prognosis, Lois was approved for an innovative treatment in Singapore, receiving the experimental cancer drug EDV. This involved twice-weekly sessions administered through a cannula over two separate seven-week cycles, with each session lasting approximately three hours.
When Lois Harris was diagnosed with neuroendocrine cancer in 2012, doctors told her there was no cure.
The rare cancer forms in glands and nerve cells throughout the body, and by the time most patients like Lois are diagnosed, it has already spread.
Chemotherapy wasn’t an option for the 73-year-old Sydneysider because it was likely to be ineffective, while targeted radiotherapy risked damaging her kidneys. Instead, she received regular injections to manage the disease and its symptoms.
After completing two rounds of the treatment, Lois was overjoyed with the results a few months later.
Luckily for Lois, the tumours have been slow growing and stable — until last year at least, when one in her liver became more active, worsening her symptoms that included an uncomfortable rash, diarrhoea, dizziness and fatigue.
With few options left, Lois turned to her long-time professional connection.
For years, she’d been working as a researcher on a new SBS documentary called Cancer Killers, about two Australian scientists developing a new cancer treatment called EnGeneIC Dream Vector (EDV).
With little left to lose, she asked them if she could trial the drug.
They approved her for treatment, which she received in Singapore through a cannula twice weekly over two seven-week cycles, each session lasting about three hours.
Lois Harris received two rounds of new cancer drug EDV in Singapore. Source: Supplied
A few months on from her second round, Lois was thrilled.
“The tumour in my liver that was acting up has decreased by about eight millimetres and I’m in stable disease again. That’s a great result for me,” she said.
Even better, there were no side effects from the treatment.
“I just went home and carried on with life. My appetite and energy were fine. I was walking 10,000 steps a day, doing pilates, going out morning and evening. It’s the complete opposite of conventional chemo.”
In Los Angeles, Anne Jonas was given EDV for her end-stage pancreatic cancer, which had left her with only weeks to live.
She said the date she received the treatment was “a big-deal day”.
“I was tired the next day but I wasn’t feeling sick, I wasn’t nauseous, I wasn’t anything. I’m like, ‘gosh, this is great, I can do this’.”
Two and a half years later, Anne’s bloods show no trace of cancer. She has become the first known person in the world with end-stage pancreatic cancer to make a complete recovery.
Anne Jonas has become the first known person in the world with end-stage pancreatic cancer to make a complete recovery. Source: SBS
Now she walks her dog on the beach and wants to run again, like she used to.
“When people ask me: ‘how are you?” I’m like: ‘I’m fine. Can you not see?’ I’m here having breakfast with you or coffee … I’m going to be a grandma.”
Hopes for a cancer cure
EDV slips inside cancer cells and destroys them from within, leaving healthy cells untouched, unlike chemo. This way, patients are able to avoid brutal side effects like nausea, vomiting or hair loss.
It’s also cheap to produce, unlike many new cancer therapies that can cost hundreds of thousands of dollars per patient, which makes it accessible worldwide.
EDV is the brainchild of Dr Himanshu Brahmbhatt and Dr Jennifer MacDiarmid, former CSIRO colleagues who were determined to find a cure for cancer.
The pair spent countless nights at a McDonald’s in western Sydney, brainstorming solutions.
“These poor fellows gave us coffee after coffee as we discussed all the problems we had to solve and blew holes into our theories,” said Brahmbhatt, who was inspired after a friend dying of cancer told him that if anyone could do something about cancer, he could.
Then one night, they realised that a solution had existed for millennia.
“Years before, under the microscope, we saw a tiny nano cell forming naturally from bacteria. We thought, if we could recreate this in large amounts and assemble a complete structure, it could solve so many of our problems,” Brahmbhatt said.
The pair left the CSIRO, set up their biotech firm, built a laboratory, and brought in a team of scientists to test their theories.
“One after the other, the theories we’d scribbled on paper turned out to be correct,” he said.
EDV entered human trials in Melbourne in 2009, with advanced-stage trials about to start in Singapore, Australia and the US, where that country’s Food and Drug Administration has granted it fast-track status to speed up approval.
Dr Himanshu Brahambhatt works at the EnGeneIC laboratory in Sydney. Source: SBS
So far, Brahmbhatt says more than 100 patients with different types of cancer have had their lives extended through EDV.
“We see cancer patients every day, many in very desperate situations. I see those eyes that are losing all hope,” Brahmbhatt said. “But this science is actually bringing people back — from their deathbeds to their families, to their lives.”
Still, like most new cancer treatments in development, there’s no guarantee EDV will ever come to market.
A long, expensive journey
EDV is just one of numerous cancer treatments being developed, with many focused on immunotherapies and targeted drugs that let doctors tailor treatment more precisely.
At any given moment, thousands of cancer trials are underway around the world.
But most will never make it to market, says Australian oncologist and clinical trial expert Georgina Long AO, joint 2024 Australian of the Year, who is developing new therapies for melanoma patients.
“It is very difficult to get cancer treatments to market because cancer is hard to treat. It is biologically one of the smartest immortal cells,” she told Insight.
“Fourteen out of every 15 drugs that get to [stage one trials] in humans are not developed further, mostly due to lack of efficacy or high toxicity.”
In the United States, only around 3 to 5 per cent of cancer drugs that enter Phase 1 trials are ever approved for use.
Dr Jim Whittle, a medical oncologist specialising in neuro-oncology at the Peter MacCallum Cancer Centre in Melbourne, said clinical trials for cancer therapies are “inherently complex”.
“Each cancer behaves differently depending on genetic and environmental factors, and this complexity demands highly specialised clinical trial design and analysis to determine which new treatments are truly an advance on the standard-of-care,” he said.
“That’s why this process can take between 10 and 15 years.”
The long timelines mean huge costs, and investors often lose patience waiting for returns.
When funding dries up, many biotech startups collapse before their treatments ever reach patients.
Brahmbhatt says his company has been on the verge of bankruptcy many times.
“Medicine takes an incredibly long time. There have been so many points where we’ve had to rebuild from scratch,” he said.
“A lot of great inventions vanish before they ever get the chance to help anyone.”
Hopes of a cure
Whittle says despite major advances in cancer therapies, the future remains unknown.
“That is part of what makes it so exciting. Many of tomorrow’s breakthroughs will come from directions we can’t yet anticipate. That’s why it’s crucial to continue supporting discovery science.”
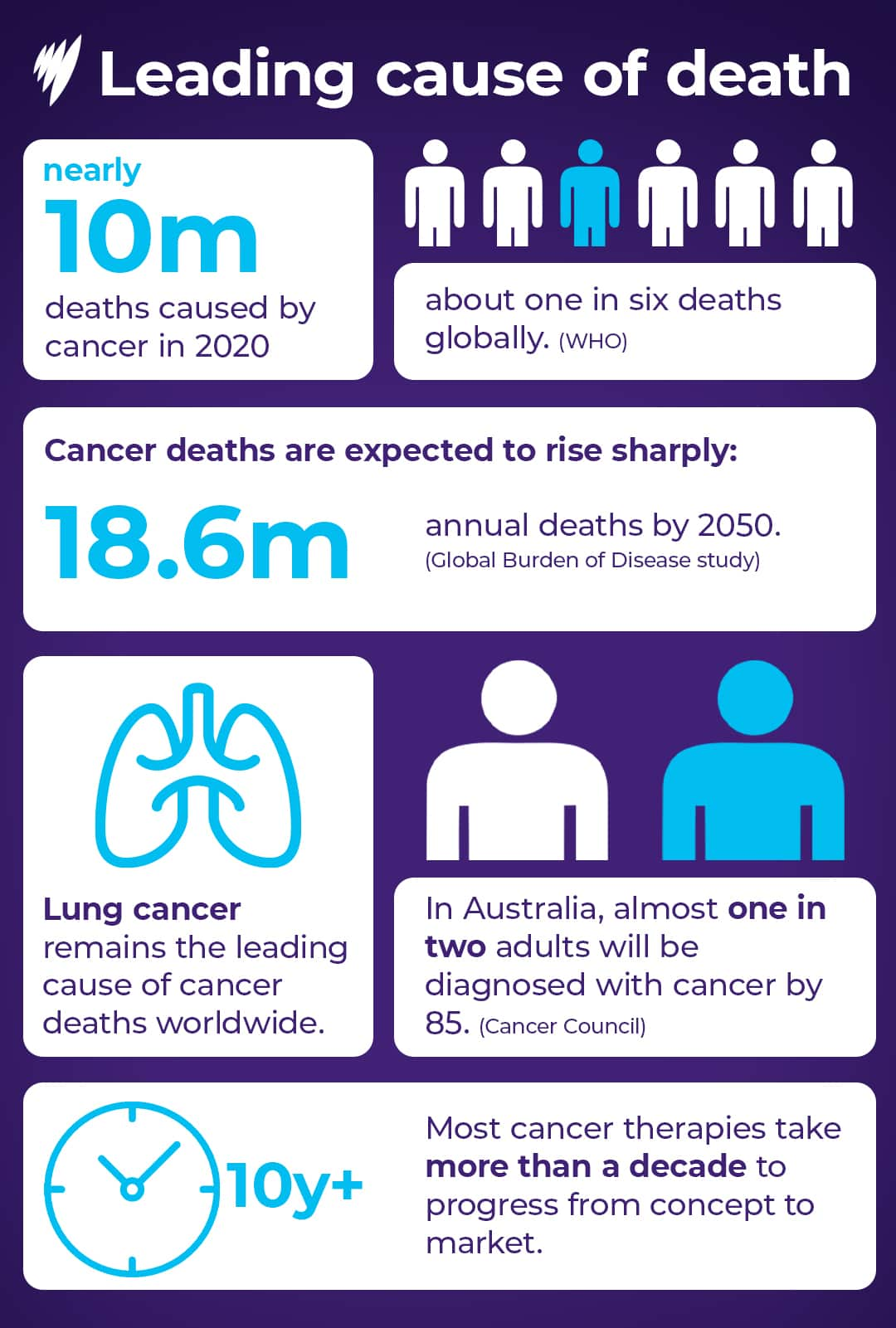
Cancer treatments can take more than a decade to progress from concept to market. Source: AAP
However, he stresses that while new drugs can target cancer cells with far greater precision — minimising the damage chemotherapy often causes to healthy tissue — chemo remains an essential part of cancer care.
“Some of the most effective cancer cures still rely on these agents,” he said.
MacDiarmid and Brahmbhatt remain determined to get their drug through the next round of trials and make it widely available for use.
“We want to see people go home cancer-free. There is no price that one can put on that,” Brahmbhatt said.
For Lois, even stable disease feels like a victory.
“If I can have another 10, 12 years of good years, that’s a huge win. At my age, things could have gone downhill fast. This promises me stable disease with fewer symptoms.
“It’s given me a chance to lead a normal life.”
For more on Australians living with unseen conditions and the ways our healthcare system can better diagnose, support and treat them, watch Insight episode Invisible Illness on SBS On Demand.